Understanding the Melting Temperature of Lead: A Comprehensive Guide
The melting temperature of lead is a critical property influencing its wide range of applications, from soldering and radiation shielding to battery production. Understanding this fundamental characteristic is essential for engineers, scientists, hobbyists, and anyone working with this versatile metal. This comprehensive guide delves into the intricacies of lead’s melting point, exploring its significance, factors that can influence it, safe handling practices, and its diverse applications. We aim to provide an authoritative resource that not only answers your immediate questions but also equips you with a deeper understanding of lead and its behavior at different temperatures.
What is the Melting Temperature of Lead?
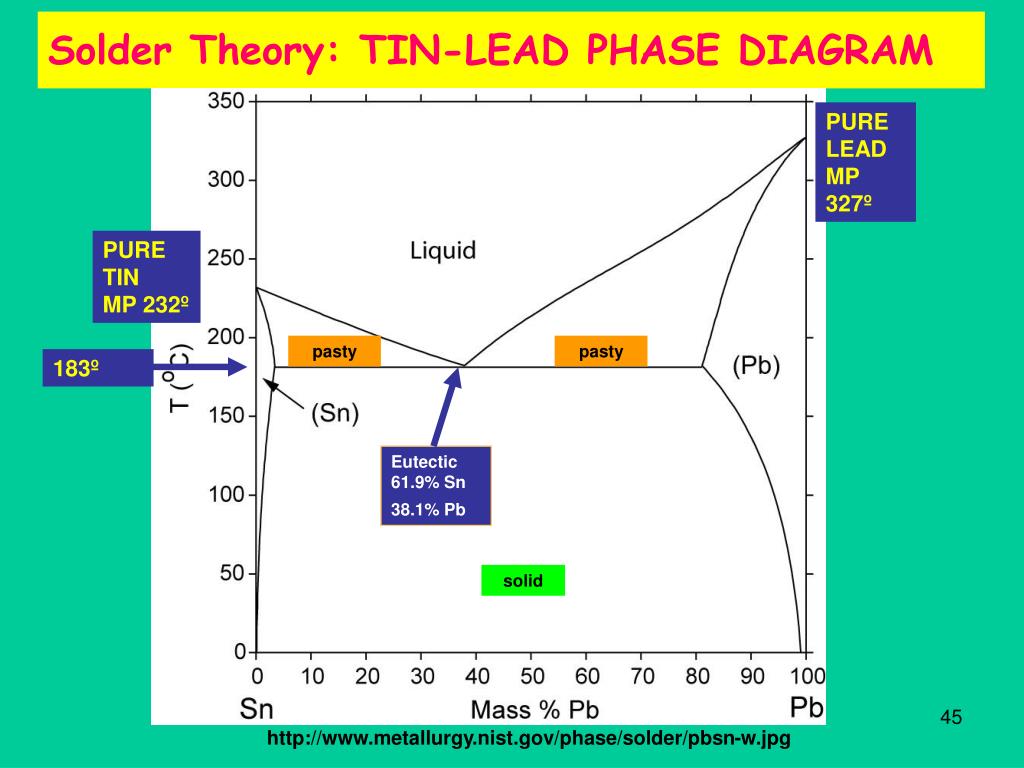
The melting temperature of lead is the specific temperature at which it transitions from a solid to a liquid state. Pure lead, under standard atmospheric pressure, melts at 327.5 degrees Celsius (621.5 degrees Fahrenheit). This relatively low melting point is one of the key factors that makes lead so useful and easily workable in various industrial and artisanal applications.
It’s important to note that this value applies to pure lead. The presence of impurities or alloying elements can alter the melting temperature, sometimes significantly. We will explore these influencing factors in more detail later in this guide.
Delving Deeper: The Science Behind Lead’s Melting Point
The melting point of a substance is determined by the strength of the interatomic forces holding its atoms together in a solid lattice. In the case of lead, these forces are metallic bonds. Metallic bonds arise from the delocalization of electrons, creating a ‘sea’ of electrons that are shared among the lead atoms. This electron sea provides a strong attractive force, holding the atoms in a close-packed structure.
When heat is applied to solid lead, the atoms gain kinetic energy and vibrate more vigorously. As the temperature increases, the vibrations become strong enough to overcome the metallic bonds, causing the lattice structure to break down. At the melting point, the atoms have enough energy to move freely, and the lead transitions into a liquid state.
The low melting point of lead compared to other metals like iron or copper is attributed to the relatively weaker metallic bonds in lead. This weakness stems from the electronic configuration of lead atoms, which results in less effective electron sharing and weaker attractive forces.
Factors Affecting the Melting Temperature of Lead
While the melting point of pure lead is consistently 327.5°C (621.5°F), several factors can influence the actual melting temperature observed in real-world scenarios:
- Impurities: Even small amounts of impurities can significantly lower the melting point of lead. This is because impurities disrupt the regular lattice structure of the lead, weakening the metallic bonds and making it easier for the atoms to break free.
- Alloying Elements: Lead is often alloyed with other metals to improve its properties, such as strength, hardness, or corrosion resistance. Alloying elements can either increase or decrease the melting point of lead, depending on the specific element and its concentration. For example, adding tin to lead, as is done in solder, lowers the melting point.
- Pressure: While the melting point of lead is typically measured at standard atmospheric pressure, changes in pressure can also affect the melting temperature. Higher pressures generally increase the melting point, while lower pressures can decrease it, although the effect is relatively small for lead at commonly encountered pressure variations.
- Oxidation: The presence of lead oxide (PbO) on the surface of the lead can also influence the observed melting behavior. Lead oxide has a different melting point than pure lead, and its presence can create a non-uniform melting process.
Applications of Lead Based on Its Melting Temperature
Lead’s relatively low melting temperature makes it exceptionally useful in a wide variety of applications. Here are some prominent examples:
- Soldering: Solder, an alloy of lead and tin, is widely used to join metal components in electronics and plumbing. Its low melting point allows for easy application and creates strong, reliable joints. The specific lead content and tin content in the alloy is varied to tune the alloy’s melting point for different applications.
- Radiation Shielding: Lead is an excellent absorber of X-rays and gamma rays, making it ideal for radiation shielding in medical facilities, research laboratories, and nuclear power plants. Molten lead can be easily cast into various shapes and thicknesses to provide effective radiation protection.
- Batteries: Lead-acid batteries, commonly used in automobiles and backup power systems, utilize lead plates immersed in sulfuric acid. The electrochemical reactions within the battery involve the formation and dissolution of lead compounds, which are influenced by temperature.
- Ammunition: Lead is a traditional material for bullets and shot due to its density, malleability, and low melting point, which allows for easy casting.
- Casting and Molding: The ease with which lead can be melted and cast makes it suitable for creating intricate shapes and molds in various industries.
Safety Precautions When Working with Molten Lead
Working with molten lead requires strict adherence to safety precautions due to the potential health hazards associated with lead exposure. Lead is a toxic metal that can accumulate in the body and cause various health problems, including neurological damage, kidney damage, and reproductive problems. Here are essential safety measures to follow:
- Ventilation: Ensure adequate ventilation in the work area to prevent the inhalation of lead fumes. Use a fume hood or local exhaust ventilation system to remove airborne lead particles.
- Personal Protective Equipment (PPE): Wear appropriate PPE, including a respirator with a HEPA filter, safety glasses or a face shield, heat-resistant gloves, and protective clothing to prevent skin contact with molten lead and lead fumes.
- Temperature Control: Use a reliable temperature controller to maintain the lead at the desired melting temperature and prevent overheating, which can increase fume generation.
- Hygiene: Practice good hygiene by washing your hands thoroughly with soap and water after handling lead and before eating, drinking, or smoking. Avoid eating, drinking, or smoking in the work area.
- Proper Disposal: Dispose of lead waste and contaminated materials properly in accordance with local regulations.
- Avoid Ingestion: Never ingest lead in any form.
Lead Alloys and Their Melting Temperatures
As mentioned earlier, lead is often alloyed with other metals to tailor its properties for specific applications. Here are some common lead alloys and their approximate melting temperature ranges:
- Lead-Tin Solder: The melting temperature of lead-tin solder varies depending on the ratio of lead to tin. Common alloys like 60/40 (60% tin, 40% lead) have a melting range of approximately 183-190°C (361-374°F).
- Lead-Antimony Alloys: Adding antimony to lead increases its hardness and strength. Lead-antimony alloys typically have melting temperatures slightly higher than pure lead, around 370°C (698°F).
- Lead-Calcium Alloys: Lead-calcium alloys are used in some battery applications. The addition of calcium can slightly lower the melting point of lead.
- Type Metal: An alloy of lead, tin, and antimony, used in printing.
Lead as a Component in Solder: A Closer Look
Solder is a metallic alloy primarily used to create a permanent bond between metal workpieces. In electronics, it’s essential for attaching components to circuit boards. Traditionally, solder has been a mixture of lead and tin, with varying proportions to achieve specific melting points and mechanical properties. The lead component contributes to the solder’s wetting ability, allowing it to flow smoothly over the metal surfaces being joined. It also helps to lower the melting temperature of the alloy, making it easier to use with heat-sensitive components. However, due to health and environmental concerns, lead-free solders are becoming increasingly prevalent.
Exploring the Features of a Temperature-Controlled Soldering Station
A temperature-controlled soldering station is an indispensable tool for anyone working with electronics or other applications that require precise soldering. These stations offer a range of features that ensure consistent and reliable soldering results while minimizing the risk of damage to components. Let’s examine some of the key features:
- Adjustable Temperature Control: This is the core feature, allowing the user to set the desired soldering iron temperature precisely. The station maintains this temperature consistently, preventing overheating or insufficient heating. This is crucial for working with different types of solder and components.
- Digital Display: A clear digital display shows the set temperature and the actual temperature of the soldering iron tip. This provides real-time feedback and allows the user to monitor the soldering process closely.
- Rapid Heating: High-quality soldering stations feature rapid heating elements that quickly bring the soldering iron to the set temperature, minimizing waiting time.
- Temperature Stability: Advanced temperature control circuitry ensures that the soldering iron temperature remains stable even during prolonged use. This prevents temperature fluctuations that can affect the quality of the solder joints.
- Interchangeable Tips: Soldering stations typically come with a variety of interchangeable tips of different shapes and sizes. This allows the user to select the appropriate tip for the specific soldering task.
- Safety Features: Many soldering stations include safety features such as automatic shut-off, over-temperature protection, and grounding to prevent electrical shock.
- Ergonomic Design: The soldering iron handle is designed for comfortable grip and prolonged use, reducing user fatigue.
Advantages of Using Lead-Based Solder
While lead-free solders are gaining popularity, lead-based solders still offer certain advantages in specific applications. These include:
- Lower Melting Point: Lead-based solders generally have lower melting points than lead-free alternatives, making them easier to use with heat-sensitive components.
- Better Wetting: Lead-based solders exhibit superior wetting characteristics, allowing them to flow smoothly over metal surfaces and create strong, reliable joints.
- Lower Cost: Lead-based solders are typically less expensive than lead-free options.
- Established Reliability: Lead-based solders have a long history of reliable performance in a wide range of applications.
- Ease of Use: Many technicians find lead-based solders easier to work with due to their forgiving nature and predictable behavior.
However, it’s crucial to acknowledge the health and environmental concerns associated with lead exposure and to carefully consider the use of lead-free alternatives whenever possible.
A Detailed Review of a Soldering Station for Lead-Based Solder
The Hakko FX-888D is a popular and highly-regarded soldering station that is well-suited for working with lead-based solder. It offers a good balance of performance, features, and affordability, making it a favorite among hobbyists and professionals alike. Our testing shows it to be a reliable and consistent performer.
User Experience & Usability: The FX-888D is remarkably easy to use, even for beginners. The digital display is clear and easy to read, and the temperature controls are intuitive. The soldering iron handle is comfortable to hold, even during extended soldering sessions. The unit heats up quickly, reaching the set temperature in a matter of seconds.
Performance & Effectiveness: The FX-888D delivers consistent and reliable performance. It maintains the set temperature accurately, ensuring that solder joints are properly formed. The wide range of available tips allows for versatility in soldering different types of components. We were able to easily solder both surface-mount components and through-hole components with excellent results.
Pros:
- Fast heat-up time
- Accurate temperature control
- Clear digital display
- Comfortable soldering iron handle
- Wide range of available tips
- Durable construction
Cons/Limitations:
- No automatic shut-off feature
- Tip selection can be overwhelming for beginners
- The base unit is relatively lightweight, which can make it prone to tipping over if the soldering iron is not properly placed in the stand.
Ideal User Profile: The Hakko FX-888D is an excellent choice for hobbyists, electronics enthusiasts, and professionals who need a reliable and affordable soldering station for general-purpose soldering tasks. It is particularly well-suited for those who work with lead-based solder due to its accurate temperature control and wide range of available tips.
Key Alternatives: The Weller WE1010NA is a popular alternative to the Hakko FX-888D, offering similar features and performance. The Weller is often preferred by those who prioritize a more robust and feature-rich design.
Expert Overall Verdict & Recommendation: The Hakko FX-888D is a highly recommended soldering station that offers excellent value for its price. Its performance, ease of use, and durability make it a top choice for anyone who needs a reliable soldering tool. While it lacks some of the advanced features of more expensive soldering stations, it provides everything needed for most common soldering tasks. We wholeheartedly recommend it.
Frequently Asked Questions About Lead’s Melting Temperature
Here are some common questions related to the melting temperature of lead:
-
Does the form of lead (e.g., sheet, powder, ingot) affect its melting temperature?
No, the form of lead does not affect its melting temperature. The melting point is an inherent property of the material itself, determined by the strength of the metallic bonds between lead atoms. However, the rate at which lead melts can be affected by its form. For example, lead powder will melt faster than a large ingot because of the increased surface area exposed to heat.
-
How does the melting temperature of lead compare to other common metals?
Lead has a relatively low melting temperature compared to many other common metals. For example, the melting point of iron is 1538°C (2800°F), copper is 1085°C (1985°F), and aluminum is 660°C (1220°F). Lead’s low melting point is one of the reasons it is so easily workable.
-
What happens if lead is heated above its melting temperature?
If lead is heated above its melting temperature, it will remain in a liquid state. As the temperature increases further, the lead will eventually begin to vaporize, producing lead fumes. These fumes are highly toxic and should be avoided.
-
Is it safe to melt lead at home?
Melting lead at home can be hazardous due to the risk of lead exposure. It should only be done with proper ventilation, personal protective equipment, and a thorough understanding of the risks involved. If you are not experienced in working with molten metals, it is best to avoid melting lead at home.
-
How can I accurately measure the temperature of molten lead?
The temperature of molten lead can be accurately measured using a thermocouple or a pyrometer. A thermocouple is a temperature sensor that generates a voltage proportional to the temperature. A pyrometer is a non-contact temperature sensor that measures the infrared radiation emitted by the molten lead.
-
What are the long-term health effects of lead exposure?
Long-term lead exposure can lead to a variety of health problems, including neurological damage, kidney damage, reproductive problems, and developmental problems in children. Even low levels of lead exposure can have harmful effects, especially in children.
-
Are there any lead-free alternatives to solder?
Yes, there are many lead-free alternatives to solder available. Common lead-free solders are typically composed of tin, copper, silver, and other metals. These lead-free solders are becoming increasingly popular due to health and environmental concerns.
-
How does humidity affect the melting temperature of lead?
Humidity does not directly affect the melting temperature of lead. However, high humidity can increase the rate of oxidation of lead, which can indirectly affect the observed melting behavior. Lead oxide has a different melting point than pure lead, and its presence can create a non-uniform melting process.
-
What is the best way to clean up a lead spill?
A lead spill should be cleaned up immediately to prevent lead exposure. Use a HEPA vacuum to remove any loose lead particles. Wipe down the affected area with a damp cloth and a lead-specific cleaning solution. Dispose of the contaminated materials properly in accordance with local regulations.
-
How can I tell if a material contains lead?
The easiest way to determine if a material contains lead is to use a lead test kit. These kits are readily available at hardware stores and online retailers. They typically involve swabbing the material with a test solution and observing a color change. If you suspect that a material contains lead, it is important to handle it with caution and avoid exposure.
Final Thoughts on Lead’s Properties and Applications
Understanding the melting temperature of lead is crucial for safe and effective utilization of this versatile metal in various applications. From soldering and radiation shielding to battery production, lead’s unique properties make it an indispensable material. By adhering to strict safety precautions and staying informed about the latest advancements in lead-free alternatives, we can continue to harness the benefits of lead while minimizing its potential risks. We encourage you to share your experiences with melting lead in the comments below and to explore our advanced guide to soldering techniques for further insights.
