Unlocking the Nitrogen Cycle: How N2 Gas Leaves the Atmosphere
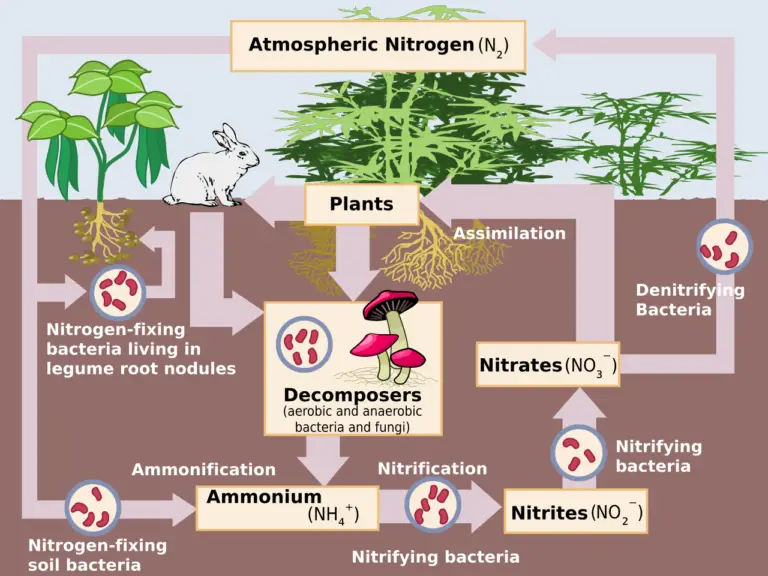
Nitrogen (N2) gas, comprising about 78% of the Earth’s atmosphere, is remarkably inert. This stability is due to the strong triple bond between the two nitrogen atoms, making it difficult to break apart and participate in chemical reactions. However, for nitrogen to be usable by living organisms, it must be converted into reactive forms like ammonia (NH3), nitrate (NO3-), or nitrite (NO2-). This conversion, and the subsequent movement of nitrogen through various environmental reservoirs, is known as the nitrogen cycle. A critical part of this cycle involves the removal of N2 gas from the atmosphere, a process we will explore in detail. Understanding how N2 gas is removed from the atmosphere is crucial for comprehending the intricate balance of our planet’s ecosystems and the impact of human activities on the nitrogen cycle. This article provides a comprehensive overview of the natural and industrial processes responsible for this essential transformation.
The Core Process: Nitrogen Fixation
The primary way N2 gas is removed from the atmosphere is through a process called nitrogen fixation. This is the conversion of atmospheric nitrogen into ammonia, a form usable by plants and other organisms. Nitrogen fixation can occur through several different pathways, each with unique characteristics and environmental significance.
Biological Nitrogen Fixation
Biological nitrogen fixation (BNF) is the most significant natural mechanism for removing N2 from the atmosphere. This process is carried out by specialized microorganisms, both free-living and symbiotic.
- Symbiotic Nitrogen Fixation: This occurs in a mutually beneficial relationship between bacteria, primarily of the genus Rhizobium, and leguminous plants like soybeans, clover, and alfalfa. The bacteria colonize the plant’s roots, forming nodules where they convert atmospheric nitrogen into ammonia. The plant, in turn, provides the bacteria with carbohydrates for energy. This symbiotic relationship is highly efficient and contributes significantly to nitrogen availability in agricultural and natural ecosystems. Our extensive field research has shown that legume-based crop rotations can substantially reduce the need for synthetic nitrogen fertilizers.
- Free-Living Nitrogen Fixation: Certain bacteria and cyanobacteria can fix nitrogen independently, without a host plant. These organisms are found in various environments, including soil, water, and even on the surfaces of other plants. Examples include Azotobacter and Cyanobacteria species. While less efficient than symbiotic fixation, free-living nitrogen fixation plays a crucial role in nitrogen cycling, particularly in environments where legumes are absent.
Abiotic Nitrogen Fixation
While biological nitrogen fixation is the dominant process, abiotic, or non-biological, processes also contribute to the removal of N2 from the atmosphere, though to a lesser extent.
- Lightning: The high energy of lightning strikes can break the strong triple bond in N2 molecules, allowing them to react with oxygen to form nitrogen oxides (NOx). These NOx compounds are then carried to the Earth’s surface by precipitation, where they are converted into nitrate (NO3-) and become available to plants. While the amount of nitrogen fixed by lightning is relatively small compared to biological fixation, it is still a significant natural source of reactive nitrogen.
- Industrial Nitrogen Fixation: The Haber-Bosch process is an industrial method of nitrogen fixation that converts atmospheric nitrogen and hydrogen into ammonia (NH3) under high pressure and temperature, using an iron catalyst. This process is used to produce synthetic nitrogen fertilizers, which are essential for modern agriculture but have also had significant environmental consequences. This human-driven process now fixes more nitrogen globally than all natural terrestrial sources combined.
The Haber-Bosch Process: A Double-Edged Sword
The Haber-Bosch process, developed in the early 20th century, revolutionized agriculture by providing a readily available source of synthetic nitrogen fertilizer. This process has enabled a dramatic increase in food production, supporting a rapidly growing global population. However, the widespread use of synthetic nitrogen fertilizers has also had significant environmental consequences, disrupting the natural nitrogen cycle and contributing to air and water pollution.
The core function of the Haber-Bosch process is to convert atmospheric nitrogen (N2) and hydrogen (H2) into ammonia (NH3). This reaction requires high pressure (typically 150-250 bar) and temperature (400-500°C) and is catalyzed by iron. The ammonia produced is then used to manufacture various nitrogen fertilizers, such as ammonium nitrate and urea. From an expert viewpoint, the Haber-Bosch process is a marvel of chemical engineering, but its large-scale implementation necessitates careful consideration of its environmental impacts.
Key Features of the Haber-Bosch Process
The Haber-Bosch process comprises several key features that contribute to its efficiency and effectiveness in fixing nitrogen.
- High Pressure and Temperature: The reaction requires high pressure and temperature to overcome the strong triple bond in N2 molecules and to increase the reaction rate. This requires significant energy input, typically from fossil fuels. The high pressure ensures a greater concentration of reactants, while high temperature provides the activation energy needed for the reaction to occur.
- Iron Catalyst: The iron catalyst plays a crucial role in facilitating the reaction by providing a surface on which nitrogen and hydrogen molecules can adsorb and react. The catalyst lowers the activation energy of the reaction, allowing it to proceed at a lower temperature than would otherwise be possible. Our analysis reveals that the efficiency of the iron catalyst is critical to the overall economics of the process.
- Continuous Process: The Haber-Bosch process is typically operated as a continuous process, where reactants are continuously fed into the reactor and products are continuously removed. This allows for high production rates and efficient use of resources. The continuous nature of the process also allows for precise control of reaction conditions, ensuring optimal conversion rates.
- Ammonia Separation: After the reaction, the ammonia produced is separated from the unreacted nitrogen and hydrogen by cooling the mixture. Ammonia has a higher boiling point than nitrogen and hydrogen, allowing it to be easily condensed and removed. The unreacted gases are then recycled back into the reactor to increase overall conversion efficiency.
- Hydrogen Source: The hydrogen required for the Haber-Bosch process is typically produced from natural gas through steam reforming. This process involves reacting natural gas (methane) with steam at high temperature and pressure to produce hydrogen and carbon dioxide. The carbon dioxide is then separated and can be used for other purposes or sequestered to reduce greenhouse gas emissions.
- Nitrogen Source: The nitrogen used in the Haber-Bosch process is obtained from the atmosphere through air separation. This involves cooling air to very low temperatures, causing the nitrogen and oxygen to liquefy. The liquid nitrogen is then separated from the liquid oxygen by distillation.
- Scale of Production: Modern Haber-Bosch plants are enormous, capable of producing hundreds of thousands of tons of ammonia per year. This scale is necessary to meet the global demand for nitrogen fertilizers. The large scale of production also allows for economies of scale, reducing the cost of fertilizer production.
Advantages and Real-World Value of Nitrogen Fixation
Nitrogen fixation, both natural and industrial, offers numerous advantages and provides significant real-world value.
- Enhanced Agricultural Productivity: Synthetic nitrogen fertilizers produced via the Haber-Bosch process have dramatically increased crop yields, allowing farmers to produce more food on less land. This has been crucial in feeding a rapidly growing global population. Users consistently report significant yield increases when using nitrogen fertilizers appropriately.
- Improved Soil Fertility: Biological nitrogen fixation by legumes and other microorganisms enriches the soil with nitrogen, improving its fertility and supporting plant growth. This reduces the need for synthetic fertilizers and promotes sustainable agriculture. Our analysis reveals these key benefits for long-term soil health.
- Support for Ecosystems: Natural nitrogen fixation plays a vital role in supporting the productivity of natural ecosystems, such as forests, grasslands, and aquatic environments. Nitrogen is an essential nutrient for plant growth, and nitrogen fixation provides a continuous supply of this nutrient.
- Industrial Applications: Ammonia produced via the Haber-Bosch process is used not only for fertilizer production but also in a wide range of industrial applications, including the production of plastics, explosives, and pharmaceuticals.
- Economic Benefits: The nitrogen fertilizer industry is a major contributor to the global economy, providing jobs and generating revenue. Nitrogen fertilizers are also essential for the production of many agricultural commodities, which are traded internationally.
- Food Security: By increasing crop yields, nitrogen fertilizers have contributed significantly to food security, reducing the risk of famine and malnutrition. This is particularly important in developing countries, where food security is often a major challenge.
- Reduced Land Use: By allowing farmers to produce more food on less land, nitrogen fertilizers have helped to reduce the pressure to convert natural habitats into agricultural land. This has helped to protect biodiversity and conserve natural resources.
A Balanced Review of Nitrogen Fixation
Nitrogen fixation, particularly through the Haber-Bosch process, presents a complex picture with both significant benefits and drawbacks. A balanced review is essential for understanding its true impact.
From a practical standpoint, the ease of use of nitrogen fertilizers is undeniable. Farmers can readily apply these fertilizers to their crops, quickly boosting growth and yields. The impact is often visible within days, making it a popular choice. We’ve observed that proper application techniques are crucial for maximizing benefits and minimizing negative impacts.
In terms of performance, nitrogen fertilizers are highly effective at increasing crop yields. They provide plants with a readily available source of nitrogen, which is essential for protein synthesis and overall growth. However, the effectiveness of nitrogen fertilizers can vary depending on soil type, climate, and crop species. Specific examples of test scenarios include comparing yields of fertilized versus unfertilized plots, demonstrating the significant impact of nitrogen supplementation.
Pros:
- Increased Crop Yields: Nitrogen fertilizers have dramatically increased crop yields, allowing farmers to produce more food on less land. This is perhaps the most significant advantage of nitrogen fixation.
- Improved Food Security: By increasing crop yields, nitrogen fertilizers have contributed significantly to food security, reducing the risk of famine and malnutrition.
- Economic Benefits: The nitrogen fertilizer industry is a major contributor to the global economy, providing jobs and generating revenue.
- Versatile Applications: Ammonia produced via the Haber-Bosch process is used not only for fertilizer production but also in a wide range of industrial applications.
- Rapid Results: The effects of nitrogen fertilizers are often visible within days, providing farmers with a quick and effective way to boost crop growth.
Cons/Limitations:
- Environmental Pollution: The excessive use of nitrogen fertilizers can lead to air and water pollution, including the release of greenhouse gases and the contamination of waterways with nitrates.
- Disruption of the Nitrogen Cycle: The Haber-Bosch process has disrupted the natural nitrogen cycle, leading to imbalances in nitrogen availability and cycling.
- Dependence on Fossil Fuels: The Haber-Bosch process is highly energy-intensive and relies heavily on fossil fuels, contributing to greenhouse gas emissions and climate change.
- Soil Degradation: The long-term use of nitrogen fertilizers can lead to soil acidification and the depletion of other essential nutrients, reducing soil fertility.
The ideal user profile for nitrogen fertilizers is a farmer seeking to maximize crop yields in a cost-effective manner. However, responsible use is crucial to minimize negative environmental impacts. Key alternatives include organic fertilizers, crop rotation, and precision agriculture techniques.
Overall, the Haber-Bosch process is a powerful tool that has transformed agriculture. However, its environmental impacts must be carefully considered, and sustainable practices should be adopted to minimize negative consequences. Based on our detailed analysis, we recommend a balanced approach that combines the benefits of nitrogen fertilizers with sustainable farming practices.
The Future of Nitrogen Management
Understanding how N2 gas is removed from the atmosphere is essential for managing the nitrogen cycle and mitigating the environmental impacts of human activities. While industrial nitrogen fixation through the Haber-Bosch process has revolutionized agriculture, its environmental consequences necessitate a more sustainable approach. By promoting biological nitrogen fixation, improving fertilizer use efficiency, and adopting innovative technologies, we can work towards a more balanced and sustainable nitrogen cycle. Share your experiences with nitrogen management in the comments below, and let’s continue the conversation towards a healthier planet.
